A cure for type 1 diabetes is close
The autoimmune disorder destroys the body’s ability to produce insulin. Some patients in experimental trials have gotten it back.
When Amanda Smith walked into the kitchen one night in 2015 to warm up a bottle for her four-month-old daughter, she couldn’t make out the numbers on the microwave. They were blurry. They’d never been blurry. Suddenly a dark thought entered her mind: diabetes. Her mom had it, and Smith was a nurse. She knew the signs. Her immune system, probably months ago, had begun destroying the cells of her pancreas that produce insulin, a hormone that enables the body to burn sugar, and now she was so low on insulin that the sugars in her blood were building up, pumping the lenses of her eyes with fluid, and distorting the light that entered.
Smith woke her husband and they raced to the hospital in Ontario, where a test confirmed her blood sugar was soaring, more than six times healthy levels. Then a doctor injected her with insulin and her levels crashed. “It was hard from the beginning,” Smith says. The diagnosis of type 1 diabetes, a chronic, incurable disease, made her angry. It made her sad. Her blood sugar crashed regularly from insulin injections, making her irritable. She’d get short with her husband. She’d get short with her three children. She felt like a burden on everyone around her. No matter how hard she tried, she felt like she failed over and over to manage her blood sugar.
But as of October, Smith hasn’t had to take supplemental insulin in more than two years. It feels, she says, like freedom.
Smith is part of the first wave of patients to receive what doctors and scientists are cautiously saying could well be, one day soon, a cure. “I never say cure,” says Kevan Herold, a physician, immunologist, and type 1 diabetes researcher at Yale School of Medicine. “But that’s the direction we’re headed in.” The breakthrough is well-timed. An estimated 9.5 million people globally have type 1 diabetes, and rates are climbing, particularly among children and young adults. Researchers do not know why.
Before the treatment, Smith, now 36, felt imprisoned in her own body, locked up in the minute-by-minute monotony of managing her blood sugar. “It sucked all around. Even to take my kid to the park, I had to make sure I had snacks in my pocket in case I got a low blood sugar.” But now, “I get so emotional sometimes about it,” she says, because even if the treatment just lasts a couple years, “these are the best years.”
Smith’s treatment is still in experimental clinical trials, results from which were published in June in the New England Journal of Medicine. The drug company running the trials, Vertex Pharmaceuticals, hopes to release the treatment this decade and predicts, pending approval by the Food and Drug Administration and European regulators, a base of some 40,000 to 60,000 customers in the United States and Europe. The treatment, called VX-880, will likely first be prescribed to a subset of type 1 diabetics who, like Smith, cannot maintain safe blood sugar levels with insulin therapy no matter how hard they try. It is not developed as a general therapy, largely because it requires immune-suppression drugs, a trade-off many diabetics won’t likely make.
But what the moment represents, scientists say, is profound. For more than a century, doctors have treated this disease by returning to the body the chemical it could no longer make: insulin. And while the discovery of insulin transformed a fatal disease into a treatable disease, type 1 diabetes is still a disease that requires constant management. Now scientists are learning to restore the very source of insulin secretion, to return the missing parts. The medicine continues to evolve; scientists are working to deliver similar treatments without having to suppress the immune system. If VX-880 continues working, researchers say, the underlying science could well cure more than diabetes.
('Type 1.5' diabetes is real—and underdiagnosed)

For most of human history, type 1 diabetes was untreatable
In ancient times, physicians noticed that the urine of certain people attracted black ants, and the ants were like an omen. Children wasted away within days of the ants’ appearance, and adults, with more fat and muscle for their bodies to eat, lived a little longer. It was as if these people were liquefying, physicians observed, flushing themselves out in their urine. They urinated constantly, and the ants were drawn to their pee because it was sticky and sweet, like honey. Shortly after the birth of Christ, the ancient Greek physician Aretaeus of Cappadocia dubbed the condition diabetes. The word means “siphon,” because, seemingly, all the nutrition of food and drink were passing right through people.
For reasons doctors still don’t understand, the immune system of type 1 diabetics begins, often in youth, spontaneously destroying the body’s sole source of insulin production, beta cells, which live within a larger cell type, islet cells, which pack the pancreas.
When someone with a fully functioning pancreas eats, say, a banana, sugar from the banana accumulates in her bloodstream, and insulin allows the sugar to enter her cells. This is the primary way humans make energy from food. Without enough healthy beta cells, the pancreas secretes little to no insulin, and sugar builds up in the blood, becoming a poison. High blood sugar damages nerves, blood vessels, tissues, and organs. Cells, meanwhile, starve. Searching for fuel, the body reaches instead for fats, and as it burns them, acid also builds up in the blood.
Globally, one in nine adults have diabetes—but most have type 2. People with type 2 diabetes often do produce insulin; but their cells don’t respond well to the hormone and cannot absorb sugar, which builds up. The underlying causes of type 2 diabetes are well understood—involving lifestyle factors, mainly, such as eating unhealthy foods or exercising too little—but for type 1, they are a mystery. What researchers do know is type 1 is most prevalent among young people in rich countries, where many patients have the money, access, and education to blunt the lethality of the disease. For example, a boy diagnosed at age 10 in the United States is likely to live to 67. But in, say, Afghanistan, that boy would probably die at 24.
(It’s possible to reverse diabetes—and even faster than you think)
For centuries, the leading treatment for type 1 diabetes was to deprive the body of any nutrients to siphon in the first place—starvation, essentially. That was humanity’s best treatment until 1921, when a young Canadian surgeon, Frederick Banting, and a medical student, Charles Best, removed the pancreas from a dog. Then they crushed the glands of the organ, froze them, ground them up, sucked them into a needle, and shot them back into the dog, which had become diabetic without its pancreas. The dog’s blood sugar dropped within two hours.
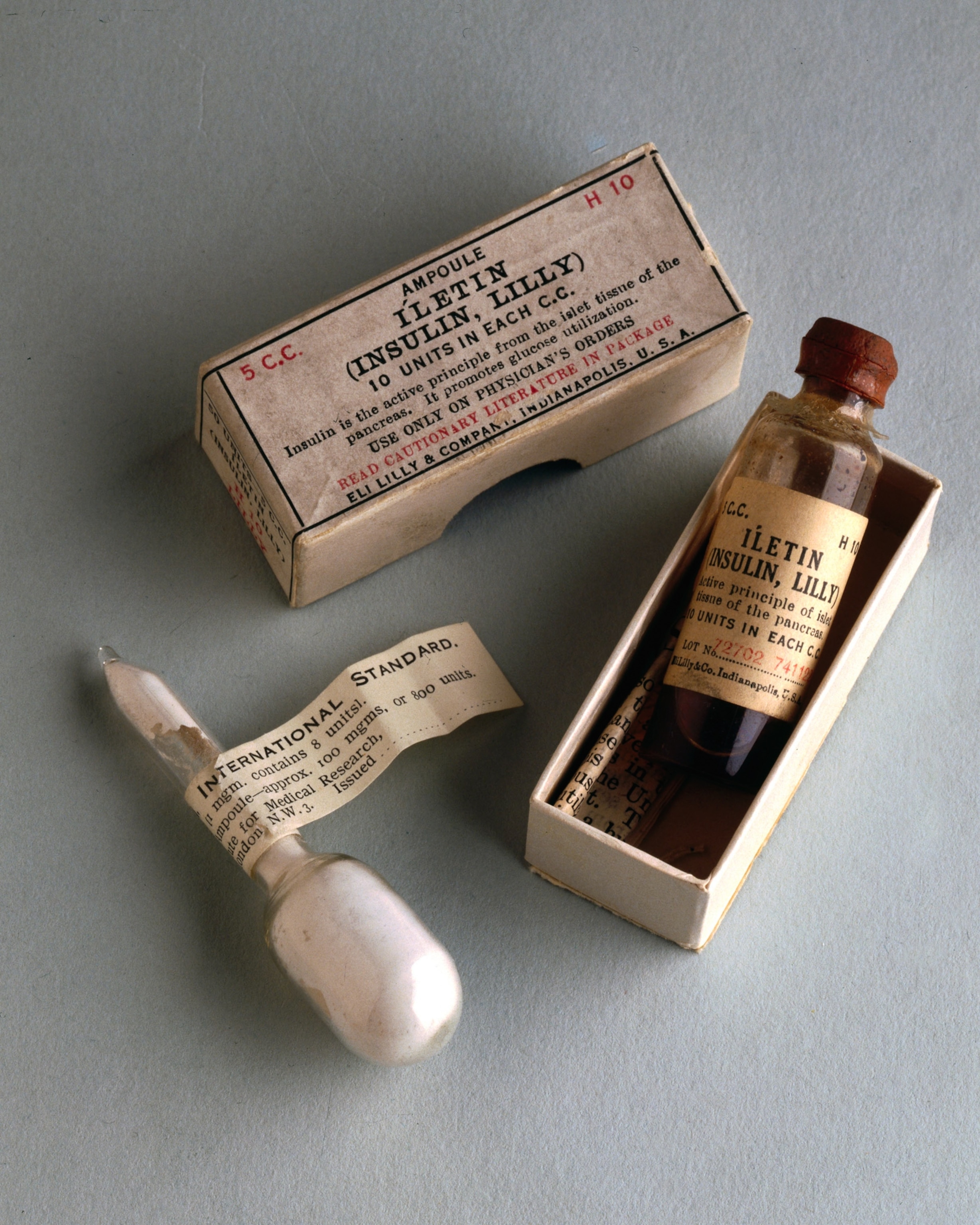
It was the first time anyone had isolated insulin, and the following year, a 14-year-old boy dying of diabetes in a Toronto hospital got an injection of insulin extracted from bovine pancreases. His blood sugar dropped within hours. Suddenly a terminal disease was treatable. The discovery saved tens of millions of lives. In the century since, doctors sometimes like to say that with therapeutic insulin, type 1 diabetics can live a “normal life.” But their definition of “normal” is a relative one, and they tend to place the onus of achieving normalcy squarely on the patient. “You can stay healthy,” a 1999 publication of the American Academy of Family Physicians admonishes, “if you control your disease.”
All the challenges of living with type 1 diabetes in the post-insulin world can be boiled down into those two letters: if. If a diabetic is able to control the disease, her life often, outwardly, can look normal. But internally it is a battle. Every day she must do the job of the beta cells herself, continuously monitoring her blood sugar with test strips or a glucose monitor. The job involves guesswork and anticipation. What’s she eaten and what will she eat? Will she exercise? Is she stressed? Dehydrated? Tired? Sick? On her period? Off her game? Her answers to these little questions help her answer the big question: How much insulin, by needle or wearable pump, does she dose? Dosing too little too often can hurt her in the future, but dosing too much can kill her where she stands. On average, a patient makes 180 decisions about type 1 diabetes a day.
Most type 1 diabetics can control the disease. Chris Binder, for example, played Division I football at the University of Maine in the 1990s. He was a kicker. From the stands, no one could tell he’d been diagnosed at the age of two—though at halftime, staff always made sure he had a big sandwich in case his blood sugar slumped. They’d say, “Is Binder’s grinder here?” Binder is 50 today, lives in New Orleans, and works at a tech company that consults with collegiate and professional sports teams to improve their performance. There isn’t much he can’t do; the disease doesn’t dictate his life. “I think I have really lived with diabetes,” he says, “and not lived for diabetes.”
But if a diabetic is unable to control the disease, her life will look different. While tying her shoes one morning before work, Jennifer Coleman, a 60-year-old accountant in Alberta, put her head through a glass cabinet as a low-blood-sugar-induced seizure whipped through her. Coleman does not get the warning signs many diabetics get when their sugar crashes—shaky knees, sweaty palms, a foggy mind, and a feeling of doom—so she’d frequently get blindsided by low lows. “It takes so much planning to do anything. That’s the biggest thing,” she says. “And you have to be prepared for bad things.”
Heather McLeod, a 59-year-old family doctor in Vancouver, was diagnosed when she was six. Even with her medical training, her blood sugar often would range wildly. “If I’m going on a walk, I better bring three juice boxes and better bring my insulin with me. Oh, do I have enough test strips? Do I have my sensors?” Like Coleman, McLeod doesn’t get the typical warnings. “It’s just living in fear,” she says. “I feel like Cinderella. I don’t know when my coach is going to turn into a pumpkin.”
It’s the same for Mary Anna Pokerznik. “People don’t understand the emotional toll of something when you work so hard and you still fail,” she says. Pokerznik, diagnosed at 11, is 55 and a retired high school math and science teacher. For years, Pokerznik was her daughter’s only ride to dance class. But often Pokerznik’s blood sugar was too low to drive, and her daughter would bawl her eyes out. “People think that with the new technology”—better insulin, better insulin pumps, better glucose monitors, better algorithms—“that’s an answer,” she says. “It really isn’t.”
Type 1 diabetes exists on a spectrum. Binder might represent the far end of one side, Coleman, McLeod, and Pokerznik the other. But the bottom line is that if anyone slips up, they can die, because even today’s most sophisticated medical devices still cannot match the precision of the tiny biological machines that come stock in the human pancreas: beta cells. That is why scientists have for decades tried to find a way to replace them.
(Eating too much of this favorite food could increase your diabetes risk)
A breakthrough decades in the making
The origins of the Vertex breakthrough trace back to one night in 1991, when Doug Melton, a 38-year-old professor of developmental biology at Harvard University, was studying the eggs of African clawed frogs in the basement of the Biological Laboratories. Melton was searching for the genes that tell the early stem cells of a single fertilized egg to transform into specialized cells. Then he got a call from his wife, Gail: Sam, their six-month-old son, was vomiting. Melton raced out of the lab. In the emergency room of Boston Children’s Hospital, he and Gail would learn their son had type 1 diabetes.
After the diagnosis, Melton gathered everyone in the lab—all 25 to 30 of his colleagues—and told them he was transitioning away from their research on frogs. Harvard had agreed to build Melton a new lab so that he could pursue a new mission: a cure for type 1 diabetes.
He wasn’t completely starting over. His work with the cells of frog embryos suggested a way forward. Those early stem cells are like biological blank slates: With the right stimuli, they can turn into any cell in the body. Melton just had to figure out the stimuli to coax human stem cells into functioning beta cells. He was already, as he puts it, “a painter, but now I was told what the subject of the painting had to be.”
Melton told me recently he had no idea how difficult the research would become, which he’s grateful for. If he’d known, he might’ve quit. “It seemed to me a simple idea,” he says: Engineer the cells his son had lost, then put them back in, all the while preventing the immune system from destroying the cells. He told his wife it would take him three to four years.
Between 1990 and 1999, Melton zipped past that three-to-four-year deadline and then zipped past it again. Melton wasn’t the only researcher looking into cell therapies, but they were each working in isolation. Back then, the scientific community was more preoccupied with trying to understand the mechanism by which insulin is secreted, not how to make the cells that do the secreting.
Then there was a breakthrough that cast their work into a new light. In a clinical trial between 1999 and 2000, University of Alberta doctor James Shapiro transplanted islet cells from cadavers into seven patients with severe type 1 diabetes. Other doctors had tried similar procedures with marginal success, but Shapiro developed a new protocol. He sourced healthy islets from multiple cadavers, processed the cells in a novel solution, and suppressed patient immune systems with steroid-free drugs. (Steroids tended to backfire in patients, making them insulin resistant and damaging their beta cells.)
Every patient quickly began producing enough of their own insulin that they no longer needed to inject it, and they continued producing insulin at follow-up more than a year later.
Pokerznik was Shapiro’s second patient in the trial. “It was hard for me to even wrap my mind around,” she says, “because I’d been diabetic for so long.” Later, Shapiro also performed the procedure on McLeod and Coleman. The women say the therapy liberated them from the minute-by-minute calculations they had to make when managing their disease using pharmaceutical insulin. It also saved them from the constant crashes. “It changed a lot,” Coleman says. “Really, for me,” McLeod says, “there was no other option.”
After the New England Journal of Medicine published the results in 2000, the University of Alberta Hospital switchboard operators were overwhelmed. Patients from all over the world wanted to get on Shapiro’s transplant list, and soon he would land a $35 million grant to do more trials.
While Shapiro’s protocol became available for patients who could not control their diabetes by other means, it was never broadly prescribed. The cadaveric islet cells required immunosuppressant drugs, which risk new infections and novel cancers. The islet cells also originate from a finite resource: deceased people. Patients typically need cells from two to three donors, and it’s not a one-time thing. The cells sometimes die, necessitating top-ups. Pokerznik, for instance, was off supplemental insulin within weeks of her first transplant in 1999. Then in 2003, she was back on insulin. In 2014: off again. In 2018: on. Since 2019, she has been off insulin.
Still, says Lori Sussel, research director at the Barbara Davis Center for Diabetes at the University of Colorado, Shapiro’s work was an inflection point: “It was a proof of concept.” When scientists saw that the islet cells were surviving in patients, they began to think that maybe Melton and the others were onto something after all. If cells from cadavers restore insulin production in patients, why can’t cells grown in a lab? “People that were not interested in stem cell therapy before,” Shapiro says, “got interested.”
Now suddenly Melton and the other researchers, who had all been working in isolation, began working together, and scientists who hadn’t thought much of their work began their own experiments. And funding followed. One year after Shapiro went public with his results, the National Institutes of Health created the Beta Cell Biology Consortium, which began awarding grants, even to researchers in Europe. As the pace of research quickened, one breakthrough begot another, and Melton began to figure out how to grow the cells.
The ‘transformational’ power of stem cells
Within each of the trillions of cells in the human body, there are some 20,000 genes, only a quarter to a half of which are expressed. Cells read genes to learn how to do what they need to do, and Melton and his team were puzzling over which genes a stem cell needed to read to learn how to secrete insulin. Which ones do they turn on in a stem cell, the scientists wondered, and which ones do they turn off? “This,” Melton says, half joking, “is an excuse for why it took us so long.”
One night in 2012, Melton’s colleagues were huddled in the lab around a sheet of plastic that looked like an enormous egg carton. It contained 96 little trays, and each tray contained a puddle of experimental beta cells engineered from stem cells. They’d mixed the solution with an antibody that would turn blue in the presence of insulin, and they’d just added sugar. No one expected anything to happen. Minutes passed. A single tray turned blue. The young scientists rushed down the hall and told Melton, who thought it finally seemed to be working. Then he said, “Repeat it.”
They did, over and over. And in the years since, Melton co-founded a biotech company to scale up production of his engineered beta cells, which a bigger biotech company, Vertex, with experience running clinical trials and applying for regulatory approvals, bought for $950 million in 2019. In a trial in 2021, Vertex gave 14 patients with severe type 1 diabetes infusions of Melton’s cells, dubbed VX-880.
Before the trial, 12 of the patients had struggled to balance their blood sugar using supplemental insulin. Ninety days out, their sugars were balanced. Two of the patients required less supplemental insulin. Ten got off it entirely. (Two patients enrolled in the trial died, though not from the treatment. Meningitis killed one; dementia killed the other.)
The scientific community is still buzzing from the publication of Vertex’s results in July in the New England Journal of Medicine. They are buzzing in part because the next month the journal published the work from another lab that, combined with a stem cell therapy like VX-880, could be the cure everyone has been hoping for.
Like Shapiro’s islet cells, patients in the Vertex trials require immunosuppression. But in the study published in August, a team from Sana Biotechnology, a smaller company exclusively working on cell therapies, showed it may not be necessary to knock down the immune system. Scientists edited three genes of the islet cells from a diseased donor, eliminating the fingerprint that marked them as foreign. Then they injected them into a 43-year-old man who was diagnosed with type 1 diabetes at age five. Six months later, the man’s immune system had not responded to the islet cells, which were still secreting insulin.
Now labs, including Vertex and Sana, are working separately on combining both technologies into a single solution: lab-grown cells that do not require immunosuppression and reliably produce insulin. Some scientists say a therapy like that would cure type 1 diabetes. Marlon Pragnell, vice president of research and science at the American Diabetes Association, says the twin breakthroughs mark a new era in medicine. “I think this is literally transformational,” he says. “Truly transformational. It’s a big deal.”
A new era in medicine is near
In the dark era before the discovery of insulin, Smith, the first patient in the Vertex trials to receive a full dose of the therapy, would have quickly died after her onset of type 1 diabetes. Now she doesn’t even need insulin therapy. “I look at it like such a blessing,” she says. Even Melton, who pioneered this latest chapter in the treatment of the disease, struggles expressing the magnitude of how far the science has come. “It’s almost like, I don’t know,” he says, “alchemy.”
Many questions remain. Will Sana’s gene-edited islet cells continue to evade the body’s immune system? Will Melton’s stem cells survive longer in humans than Shapiro’s cadaveric islets? Time is needed. Meanwhile, the advances in the parallel fields of stem cell therapy and gene editing are enabling breakthroughs in the treatment of other diseases.
For instance, in an experimental clinical trial that began in 2018, a surgeon at Mass Eye and Ear transplanted a stem cell therapy into the eyes of 14 patients with badly damaged corneas. Many had no functional vision, and their eyes hurt badly. At checkups 18 months later, the surgeon found that the graft had fully repaired the cornea of 10 patients, improving their vision and reducing their pain. Nature Communications published the findings in March. Then in May, the New England Journal of Medicine published the results from scientists at the Children’s Hospital of Philadelphia and University of Pennsylvania’s Perelman School of Medicine, who gave an infant a gene-editing therapy to treat a rare, often lethal disease, and the therapy appears to be working.
Vertex for now is leading the efforts to cure type 1 diabetes. The company is in the final phase of clinical trials for its stem cell therapy and anticipates results next year, after which it will submit for Food and Drug Administration approval. Vertex hopes to release the therapy onto the market by the end of the decade. Although the company has not yet settled on pricing, VX-880 is likely to be extraordinarily expensive. (The company initially listed a recent blockbuster therapy for sickle cell anemia at $2.2 million. )
While the market for the therapy is likely to be limited to the most severe cases, in time, it could grow. The Centers for Disease Control and Prevention hasn’t been tracking the prevalence of type 1 diabetes for very long, but its latest figures set the number of Americans younger than 20 with the disease at 304,000. Modeling suggests that number will climb 65 percent by 2060.
Smith still gets served the kinds of advertisements on Facebook and Instagram that tell her eating cinnamon, say, will cure her type 1 diabetes. She never thought there could be an actual cure in her lifetime. To be clear, she says, VX-880 is not it, not yet. “We don’t know how long it will last,” she says, but she feels healthier now on immunosuppressants than she did when her blood sugar swung wildly—a sentiment shared by many of Shapiro’s patients. Smith takes three pills three times a day, and they’re tiny pills. “It’s nothing,” she says.
The medical community is unlikely to broadly recommend cell therapies such as VX-880 when they hit the market because of concerns about the risks of immune suppression, which is frustrating for patients who say they deserve the autonomy to decide. Binder learned about VX-880 in his interview with National Geographic. If he was a candidate for the therapy, he’d be interested, he says, even though he balances his blood sugar well with an insulin pump and glucose monitor. “For anybody who’s lived for their entire lives with this illness, to be free of it,” he says, “that has got to be intriguing.”