

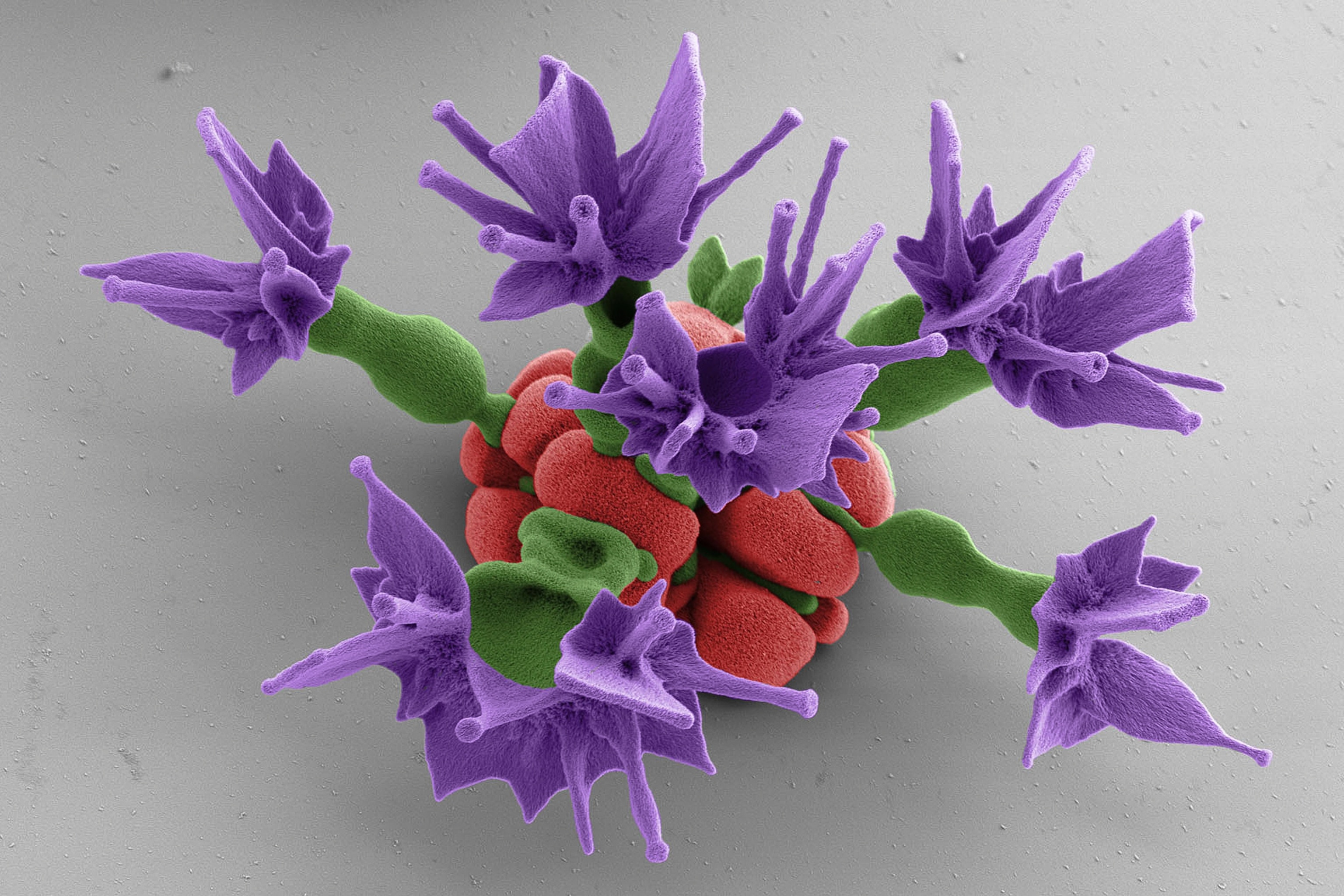

A flower fit for a Lilliputian maiden, this microscopic "rose" was grown in a laboratory at Harvard University using a solution of chemicals and minerals.
"[This] is the very first image I shot," said Wim Noorduin, a researcher specializing in crystal growth at Harvard.
Building micro- and nano-size particles—some much smaller than the width of a human hair—is a huge field, since they have potential uses in optics and engineering, said Noorduin.
Getting structures to self-assemble or grow from a solution of chemicals is relatively straightforward: Confectioners have done this for years when growing rock candy.But the trick with Noorduin's technique, which he has taken years to perfect, is he can control the shapes of the structures as they're growing by changing the temperature, pH, and carbon dioxide content of his chemical solutions. (Related:"Pictures: Best Micro-Photos of 2012.")Researchers chose to create "flowers," "stems," and "vases" because they were the easiest shapes to start with.
"You can collaborate with the process as it's [happening]," said Noorduin, who published his research May 16 in the journal Science.
All you need is a beaker of water mixed with barium salts and sodium silicate, a flat plate to place inside the beaker for the flowers to grow on, and a lid.
—Jane J. Lee
Pictures: Nano "Flowers" Created in Lab
Scientists can control the self-assembly of molecules to build nano-size flowers in the lab, a new study says.