Ebola Strikes Mali Just as Vaccination Effort Gets Under Way
An imam crosses the border while ill with Ebola, potentially sparking an outbreak.
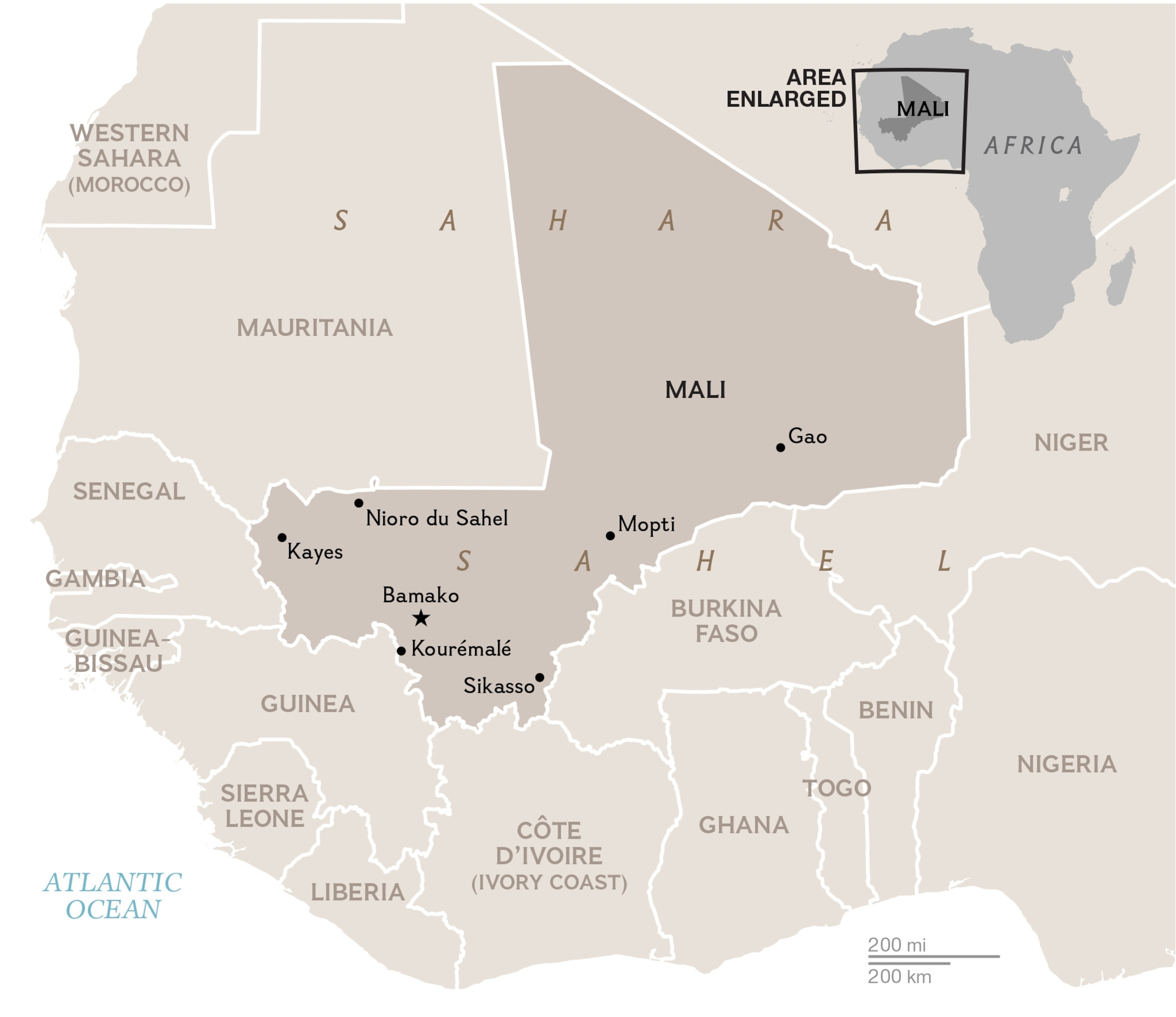
Public health officials in Mali are scrambling to trace over 200 people who may have been in contact with a religious leader who died of Ebola there this week. The 70-year-old imam had crossed the border from Guinea, where the current West African outbreak began last December and has now infected more than 14,000 and killed at least 5,000. (Related: "From Senegal and Nigeria, 4 Lessons on How to Stop Ebola.")
His caregivers in Bamako, Mali's capital, failed to recognize that he was infected with the deadly virus, and many people came to visit the religious leader while he was ill. After his death, his body was cleaned, again without realizing the risk for contagion.
His burial in the border town of Kourémalé, Guinea, was well attended. A nurse who treated him has already died, and a technician who gave him a sonogram is sick with the disease. Several other people who came in contact with the imam have died of unknown causes, including family members who rode in the car he was transported in.
Ironically, the cluster of new cases comes at the same time that an experimental Ebola vaccine is being tested on health care workers in Mali. Myron Levine, director of the Center for Vaccine Development at the University of Maryland's School of Medicine, has spent the past four decades developing, improving, and piloting new vaccines. Now Levine is collaborating with health officials in Mali to test the new Ebola vaccine. He talked with National Geographic about the new outbreak and the progress of the vaccine trials.
Why are we seeing Ebola in Mali now?
Mali shares a very long border with Guinea. It's a porous border. On the main crossing points there's control, but in the hinterland, anyone can go from Mali to Guinea and back. The countries are extremely similar. The tribal languages and customs—the difference in nationality is pretty artificial for many parts along the border. (See "Mapping the Spread of Ebola.")
So, is a better question how have they managed to avoid it for this long?
We have been expecting for months and months that sooner or later there would be importations and some disease.
Mali seemed to have handled its one previous case well, tracing more than a hundred people who had come into contact with a sick two-year-old and tracking them for three weeks to confirm they did not fall ill.
We were feeling quite good about this. Then this other importation occurred, which is much more complex. It's really a Murphy's Law-type importation.
So what could go wrong, did go wrong. Not only was the victim's infection undetected, but he was someone whose body would likely draw contact after his death.
All bad luck. The planets are not well aligned on this one.

A situation like this would seem to underscore the urgency of finding a vaccine. I understand you were working with colleagues here in Mali testing a promising one when this latest outbreak occurred.
It's one of two leading candidates. It's the one associated with a big pharmaceutical vaccine manufacturer [GlaxoSmithKline], which has been able to tweak the production and manufacturing methods. (Related: "Long Quest for Ebola Vaccine Slowed by Science, Ethics, Politics.")
It's being tested now in people for the first time?
In the middle of August it had never been in a human being, but it had protected monkeys against a 100 percent lethal challenge of Ebola Zaire [the strain of virus responsible for the outbreak in West Africa]. Because of that, there was great excitement to get this vaccine tested.
First it was tested in healthy people in the United States and Europe, and now you've moved on to Mali. How many people have you vaccinated there?
As of Monday, we have immunized 50 subjects with three different dosage levels. We're waiting for more vaccine to come in. Had it arrived when we hoped, we would have been near 80 subjects. But it's been delayed several days now coming in. It's been a tough week in Mali.
After someone is vaccinated, how do you tell in healthy people whether the vaccine is effective?
We're looking for an antibody against a key virulence protein of the Ebola virus. When [specimens from all 80 people who have been vaccinated] are tested, it will be possible to make a final decision about what the dosage levels should be for field trials and future manufacture.
So far, you've seen no significant side effects from the vaccine?
No. [We] haven't.
You're also looking to see which dose is most effective?
It's the type of vaccine where a weakened virus that cannot replicate in a human being is used, but it's live. By being alive, the nonspecific immune system sees it as a pathogen, a bad germ, and mounts a nonspecific defense. The higher the dose you give, the stronger the immune response. We have tested moderate doses. We also want to use the lowest possible dose so we'll have enough to vaccinate more people.
And you'll be vaccinating health care workers first?
All the subjects are health care workers. In the three main affected countries, 10 percent of the deaths have been in health care workers. If there were some slip in the use of personal protective equipment (PPE) that health care workers wear when they're in contact with Ebola patients, we hope the vaccine can be life-saving.
That was always the goal when we started in August, to be able to come up with extra protection for this cadre of people. It doesn't mean they aren't going to use PPE, but we'll give them a bit of confidence or courage or hope that they have something more. (See "Doctors and Nurses Risk Everything to Fight Ebola in West Africa.")
Will the vaccine also work to help people who know they've already been exposed—like family members or health care workers?
With the other leading vaccine, when someone inadvertently had a needle stick with a needle contaminated with Ebola, that person was given this other vaccine immediately and didn't get Ebola. But it's hard to know whether that was a real challenge [whether they would have developed Ebola without the vaccine] or not.
With the vaccine we're working with, we don't yet know whether giving it during incubation—after exposure but before there's onset of disease—whether you can build up enough immunity fast enough to be able to preclude onset of clinical disease. (Related: "Tracking a Serial Killer: Could Ebola Mutate to Become More Deadly?")
When you started the vaccine trial in Mali, you didn't know there would also be an Ebola outbreak there. How is the arrival of Ebola affecting your research and vice versa?
The person who drew the blood from the two-year-old [to confirm that she had Ebola], that doctor was the first vaccinee. A couple of weeks [after his vaccination,] this imported case came and we had to send someone up country to draw the blood. If you've got to send someone, why not send someone who, in addition to knowing how to use PPE, also is vaccinated?
So these vaccinated health care workers may be able to make a difference if more cases are reported in Mali?
Eighty vaccinations gives us a cadre that's big enough to be able to use them for treatment of Ebola patients and contact tracing where the contacts may be ill already or on their way.
Why did you choose to do the trial in Mali?
Originally for the reason that there was no Ebola in Mali and there was the possibility that it could or would cross the border. That would allow the very first trials to be done without any chance of contact with the Ebola virus.
During this testing phase, you need to make sure that the people you're vaccinating aren't already infected?
Having a population without subclinical infections that could confuse the readouts was why Mali was initially so attractive.
Do you worry that even if the vaccine is proved effective that we won't have enough supply? Can drug manufacturer GSK make enough of it to have an effect?
This is a disease where you would not expect to immunize the entire population, like you do with measles or [meningitis], where everyone is pretty much at risk. There's amplified transmission in health care settings, with traditional burials, and when you are in the home of a case taking care of someone.
A relatively small amount of vaccine used in a very propitious way could conceivably interrupt transmission if—and that's capital I, capital F, underlined and bolded—the vaccine shows efficacy similar to what was seen in monkeys. We'll know for sure by the end of December.
This interview has been edited and condensed.