Step outside, and a vast array of multicellular life greets you wherever you go—from the mushrooms and trees of our forests to the fish and seaweeds of our oceans. But for much of life’s more than 3.5-billion-year run on Earth, the complexity that we see in today’s plants, animals, algae, and fungi just didn’t exist.
How did life evolve from single cells into today’s “endless forms most beautiful,” as Charles Darwin put it? How did cells first glom together, learn to cooperate, and yield organisms that contain millions, billions, or even trillions of cells?
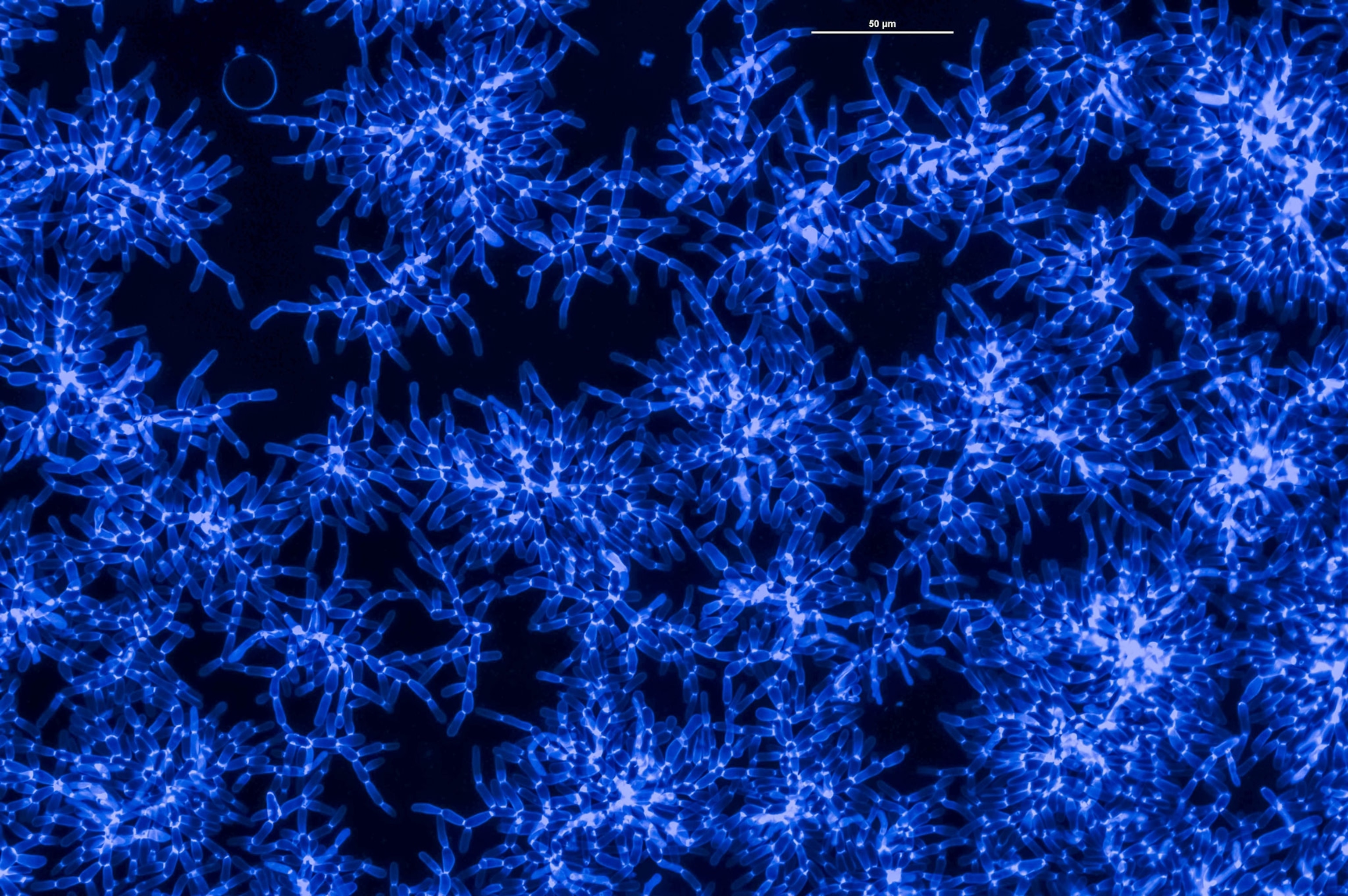
Scientists still don’t know how this transformation occurred, but thanks to yeast, a humble fungus, they might be getting closer to solving the puzzle. In a new experiment, researchers at the Georgia Institute of Technology oversaw the evolution of clusters of yeast that each grew to contain hundreds of thousands of cells—the largest of their kind—enabling the scientists to study the possible origins of complex multicellular structures.
The new research, which has been submitted to the journal Nature and posted online as a preprint, produced clumps of yeast cells a couple millimeters across that each hold roughly 450,000 cells, the largest of their kind. In the process, the yeast also went from being a hundred times weaker than gelatin to being as hard as wood—the result of the yeast evolving large, entangled networks of cells. “Everything has been a surprise,” says study co-author Will Ratcliff, an evolutionary biologist at Georgia Tech. “This is the coolest paper we’ve ever written.”

The dramatic transformations in the yeast underscore natural selection’s power to shape life. And since the experiment is slated to go on for years, future scientists studying the descendants of these yeast clusters may uncover the keys to forming ever more complex types of organisms.
One cell, two cell, fed cell, grew cell
Throughout evolutionary history, simple multicellular organisms comprised of balls or sheets of a few hundred cells have popped up fairly often, such as the green algae Volvox, which forms hollow, globe-like colonies. But complex multicellular organisms have arisen just six times on the tree of life: plants, animals, two groups of fungi, and two groups of algae.
The Georgia Tech yeast doesn’t have this level of complexity, as each cluster contains only one kind of yeast cell rather than the many types of tissues that comprise complex life. But the lab model could represent early multicellular organisms that were just figuring out how to hold many cells together without falling apart, helping to reveal the basic rules behind these evolutionary leaps.
Experimental attempts to model evolution in the lab go back more than a century. The most famous of these experiments, run since the 1980s by Michigan State University biologist Richard Lenski, has watched E. coli evolve over more than 60,000 generations. Along the way, some of these bacteria have gained the ability to eat citrate in the presence of oxygen, which E. coli almost never does in the wild.
Inspired by Lenski, Ratcliff has spent a decade probing the origins of multicellularity with yeast. In 2012, he announced promising findings with a mutant strain of “snowflake” yeast. In this strain, budding daughter cells don’t easily break off from their parents, which results in small clusters of branching cells, hence the name. By the time these clusters amass a couple hundred cells, however, they become unwieldy enough to break in two.
It wasn’t an accident—it was an outcome of natural selection.
G. Ozan Bozdag, Postdoctoral researcher
Life has several routes to evolving simple multicellularity, as seen with the snowflake yeast. But the real question is how to get stable clusters of cells numbering in the hundreds of thousands or more, says Harvard University paleontologist Andy Knoll, an expert on the origins of multicellularity who wasn’t involved with the new study. Getting so many cells to stick together would have been a crucial step in the evolution of complex multicellular life—so Ratcliff looked for ways to grow bigger clusters of yeast.
Natural selection in a test tube
To get evolution working in the lab, Ratcliff and his colleagues grew the yeast in constantly shaking incubators to keep it mixed-up and moving. Every day, the researchers pulled a random tenth of the yeasty fluid out of a test tube and placed it in a fresh tube. The yeast clusters within this smaller sample were then left to settle to the bottom of the tube for five minutes. The bigger the yeast cluster, the faster it settled out.
The scientists used only the bottom of the settled yeast—that is, the biggest clusters—within each generation to seed the next generation. This procedure placed a huge evolutionary pressure on the yeast to make the biggest clusters possible.
But from 2012 and 2016, Ratcliff kept running into a wall. For the first couple months of an experimental run, the yeast clusters would get bigger, but then they would top out at only 300 to 400 cells. Ratcliff began to suspect that the system was self-limiting, somehow.
The key to providing the right evolutionary pressures to the yeast to get the clusters to grow bigger arrived when postdoctoral researcher G. Ozan Bozdag joined Ratcliff’s lab and suggested growing the yeast at different oxygen levels. Throughout life’s history, oxygen levels in Earth’s atmosphere have varied dramatically, with potentially significant effects on how and when multicellular life evolved. So Bozdag and Ratcliff ran the experiment with zero, partial, and full levels of oxygen.
The three-track experiment, which began at the end of 2016, used five replica lineages in each oxygen treatment. The clusters’ sizes grew gradually at first and stalled—until about 200 days into the experiment, when one of the no-oxygen lineages started to show a few clusters big enough to see with the naked eye. Then the other four lineages without oxygen grew visible clusters as well.
Bozdag thought the clusters were “just an accident, a chance event,” at first. But after running the experiment multiple times, he began to realize that “it wasn’t an accident—it was an outcome of natural selection.”
After 600 days, the yeast clusters that grew without oxygen expanded to contain an average of 450,000 cells each. The surprising result suggests that in the early days of multicellular life, oxygen may have been a hindrance to some developing organisms.
Cellular modifications
As the experiment continued to run, Bozdag and Ratcliff watched as the cells of the biggest clusters began to look more elongated, very different from their nearly spherical ancestors. The evolved clusters’ cells also had bigger points of contact to the cells that budded them, likely strengthening the clusters’ branches.
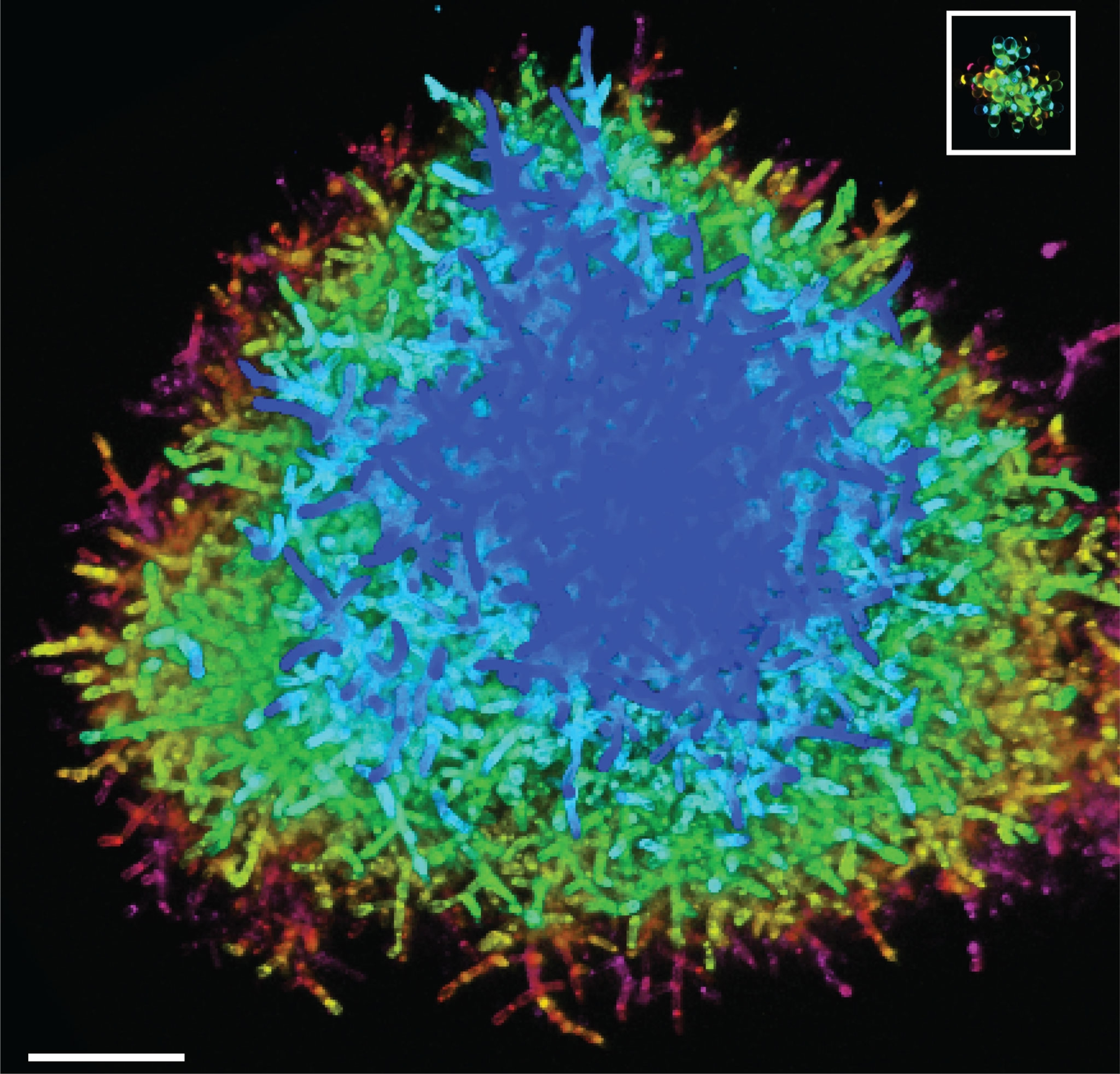
The scientists were surprised to find that the evolved yeast clusters were as tough as wood, much stronger than predicted. At first, the reason for this enhanced strength was a total mystery.
After months of work, Seyed Alireza Zamani-Dahaj, a co-author of the new study and former Ph.D. student at Georgia Tech, took vials of the beastly yeast to a powerful scanning electron microscope at the University of Illinois Urbana-Champaign. He embedded the yeast clusters in resin and used an extremely fine diamond blade to gradually expose layers to scan with the microscope.
It took six months for Zamani-Dahaj to transform his massive stack of 2D images into a 3D model of a cluster’s interior. But when he did, he realized that the clusters’ cell branches were deeply entangled—making it much harder for any one break within a branch to fracture the overall structure.
Letting life roll
While the evolving yeast blobs at Georgia Tech provide an exciting new tool to probe the origins of complex life, they do not represent the exact way multicellular life arose. Plants and people didn’t evolve from yeast, which are themselves highly evolved fungi, rather than the basic cells that first clustered together billions of years ago.
Modern yeast’s metabolism without oxygen may also not reflect how the earliest complex multicellular life operated. Knoll, the Harvard paleontologist, notes that the ancestors of modern fermenting yeast appear to have required oxygen to live, with their descendants probably evolving fermentation in response to the arrival of flowering plants—and their highly sugary fruits.
Tiffany Taylor, an evolutionary biologist at the United Kingdom’s University of Bath who wasn’t involved with the study, says that the experiment’s real strength will lie in letting it run for decades, like Lenski’s work in E. coli. Only then will evolution take the yeast into truly unexpected territory.
As the yeast clusters get bigger, access to resources within each cluster will become more and more of an issue, and cells deep in the interior will risk starving. Will the yeast clusters stumble upon mutations that let them form pores or channels so nutrients can diffuse into the cores? Eventually, will the yeast develop different types of cells, each with specialized tasks, like a true multicellular organism?
Nobody knows, including Ratcliff and Bozdag. The only way to find out is to make a career-long commitment—and let the experiment rip for years, if not decades, to come.
“Not a lot of people want to do a 30-year-long evolutionary experiment,” Ratcliff says. “But I think the payoff here is huge.”