Error undermines finding of health risks in first gene-edited babies
The 2018 birth of two gene-edited babies shocked the world. However, a study suggesting they have a risk of early death has now been retracted.
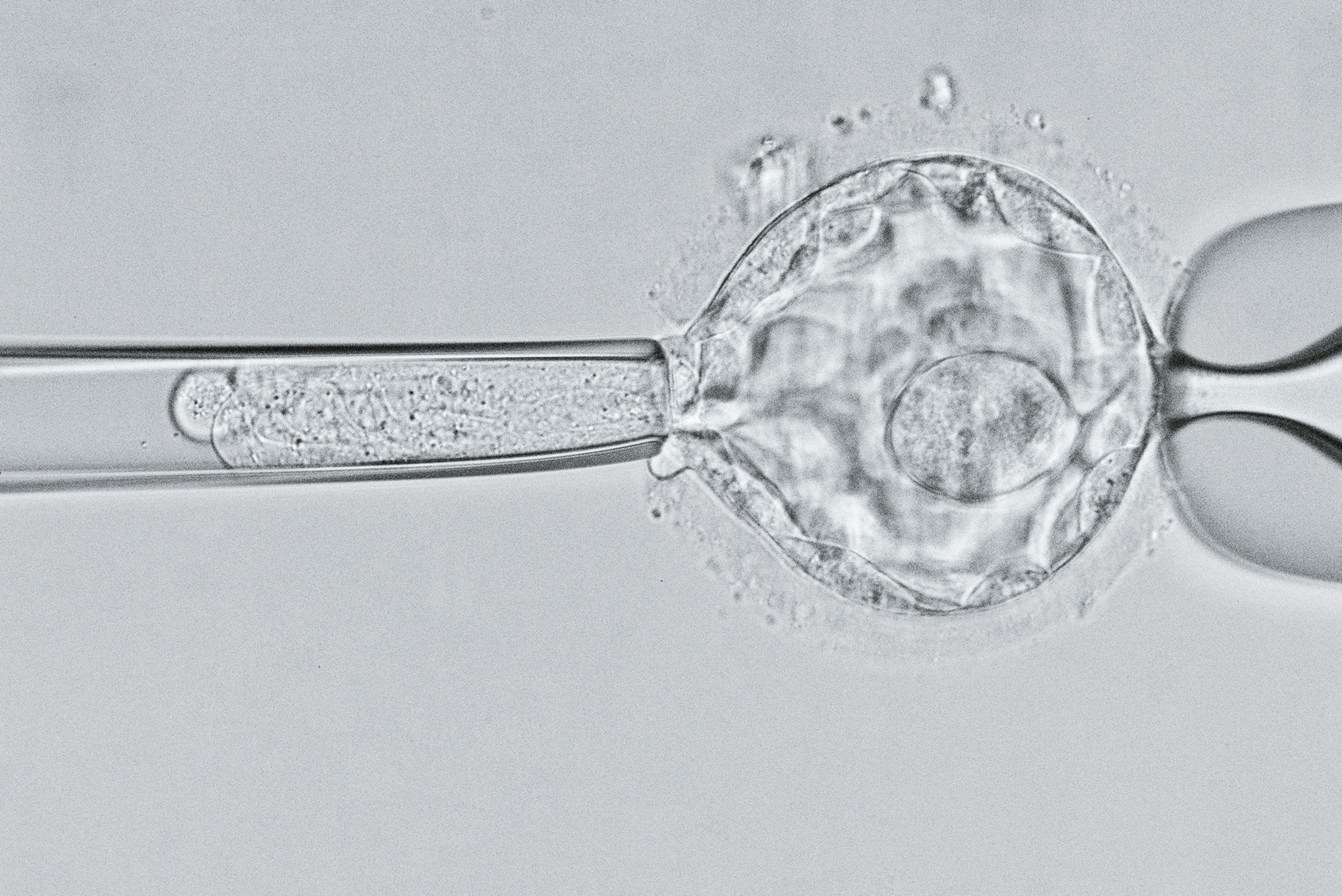
Chinese researcher Jiankui He shocked the global medical community in November 2018 when he announced the birth of two babies whose DNA he had edited—the first ever humans born with heritable changes to their genomes, made using a technique called Crispr-Cas9. He says he made the changes to lower the babies' risk of contracting HIV, but the news instantly sparked ethical and medical controversy about his work, and about the use of gene editing in humans.
Now, a study finds that the edits may have actually hurt the babies' lifespans.
As they report today in the journal Nature Medicine, researchers analyzed a U.K. genetic database and found that when people naturally have a trait similar to the one that He engineered into the babies' DNA, they have about a 21 percent greater risk of dying before the age of 76 than people who don't have this trait.
“There might be a perception that when you have one mutation, you have one effect. But in fact, one mutation might have many different effects,” says study coauthor Rasmus Nielsen, a biologist at the University of California, Berkeley.
“When we think about genetic engineering in humans, one of the many things we should consider is that the consequences can be hard to predict—that a mutation that's beneficial in one context is very detrimental in other contexts,” he says. (Find out about the first human embryos that were edited in the U.S.)
Heightened risk
In his public 2018 announcement, He said that his goal was to confer resistance to HIV, or human immunodeficiency virus. He did this by editing mutations into the CCR5 gene, which encodes a receptor on the outside of immune cells, making it an important player in how the immune system behaves. One of the most widely studied variants of this gene is the ∆32 variant, which is shorter than normal and is essentially broken. This breakage provides protection from HIV, since the virus infects immune cells by latching onto the protein that the functional CCR5 gene encodes.
But treatments for HIV have come a long way, and many experts argued at the time that this procedure was medically unnecessary. What's more, other pathogens thrive when CCR5 is broken, creating other risk factors. For instance, one 2015 study showed that having one or two copies of CCR5-∆32 nearly quadrupled a person's odds of dying from influenza.
While the mutations He created aren't perfectly identical to the ∆32 variant, it seems that the babies' CCR5 genes are broken in a similar way. To find out more about what that might mean for them, Nielsen and Berkeley postdoctoral researcher Xinzhu Wei combed through nearly 410,000 genomes in the UK Biobank, a volunteer DNA archive, looking for the fates of people who naturally have two copies of CCR5-∆32.
To account for sampling biases, Wei and Nielsen compared a thousand randomly generated subsets of the data against each other. When they did, they found that overall, having two copies of CCR5-∆32 increased a person's risk of dying before the age of 76 by three to 46 percent, with an average risk increase of 21 percent.
Wei and Nielsen caution that their work shouldn't be over-interpreted, in part because today's DNA databases are geographically biased. The pair's research is based on the genomes of U.K., not Chinese, volunteers. Similar biases plague the research that He used to try and justify editing the babies' DNA in the first place: Prior studies found signs of CCR5-∆32's protective effects against HIV in European, not East Asian, populations.
“I would like to make the message clear that the effect of the mutation depends on the genetic background and the environments, and that we don't have information to speculate about its effect in East Asians,” Wei, the study's lead author, says in an email.
Lasting effects
The new findings are bound to refocus attention on the ethical quandaries raised by He's research. Before and since He's announcement, researchers around the world have been calling for a moratorium on heritable edits to the human genome. In an essay published in Nature in May, a group of prominent Chinese bioethicists denounced He and called for a total revamp of China's governance of research ethics. In January, He was fired from the Southern University of Science and Technology in Shenzhen, China.
The difference is as big as modifying the entire operating system of a computer, as opposed to modifying one single [piece of] software installed for a particular task.Xinzhu Wei, University of California, Berkeley
Researchers are careful to point out that debate over He's work shouldn't discount Crispr's transformative medical potential, since the gene-editing technique doesn't make heritable changes in many therapeutic applications. For example, to treat a genetic disease, scientists can harvest cells from a person's organ, repair those cells' genes with Crispr, and reintroduce the modified cells to that organ to repopulate it. Similarly, researchers could use CCR5-∆32 to help treat HIV by modifying the immune cells of someone who already has HIV to make them more resistant to the virus. (Work with Crispr in mice is also advancing the ability of same sex couples to have biological children.)
When He edited the baby girls' genomes, however, he did so when they were just fertilized eggs—which meant that his edits are present in nearly every cell in their bodies, including their eggs. If these babies, nicknamed Lulu and Nana, decide to have children later in life with their unaltered eggs, their offspring are guaranteed to have at least one broken copy of CCR5.
“The difference is as big as modifying the entire operating system of a computer, as opposed to modifying one single [piece of] software installed for a particular task,” Wei says in an email. “Most of the time, I think, people who encounter a computer problem would not choose to fix a problem by renovating the operating system when it could be resolved in alternative ways.”
Crispr also isn't 100-percent accurate, so it's possible that other genes in the babies have been altered, with unclear and unintended consequences down the line. It may well take decades to see how Lulu and Nana fare. In Nature, the Chinese bioethicist group argued that procedures must be in place to monitor and care for the duo for the rest of their lives.
But even now, the babies provide a profound reminder for global caution, as Crispr co-discoverer Jennifer Doudna, a geneticist at the University of California, Berkeley, wrote in April for TIME Magazine.
“As the scientific community now works to establish stronger safeguards,” Doudna wrote, “He’s fateful decision to ignore the basic medical mantra of 'do no harm' and risk the unintended consequences will likely be remembered as one of the most shocking misapplications of any scientific tool in our history.”