Elusive Quest for One True Kilogram Finally Pays Off
The international system of measurement has a weight problem—and a crystal ball could provide the solution.
For over a century, a golf-ball sized cylinder locked in a vault in Sèvres, France, has defined the true mass of a kilogram (2.2 pounds) for the entire planet.
The International Prototype of the Kilogram, a gleaming object of platinum and iridium otherwise known as Le Grand K or Big K, is the last of its kind. All other standardized units of measurement have been redefined in terms of a fundamental natural constant. For instance, the meter, which was originally represented by a metal bar, was redefined in 1983 as the distance light travels in a vacuum during 1/299,792,458 of a second.
An international cohort of scientists hopes to do the same for the kilogram and, once and for all, toss out these archaic ways and restore balance to the global system of measurement. This week, they announced the results of a study that might ultimately end the wait for a new weight by calculating the most accurate measure yet of Avogadro’s number—a heap of atoms more numerous than there are stars in the universe. That number is the bridge between the atomic world and the visible world.
The Incredible Shrinking Kilogram
The origin story of Big K reads like a fairytale. The cylinder-shaped artifact was forged under the guidance of the General Conference on Weights and Measures (CGPM), which stated in 1889, as if by royal decree: "This prototype shall henceforth be considered to be the unit of mass."
For over a century, the kilogram was sealed within three glass bell jars beneath the International Bureau of Weights and Measures, where it was protected from dust, moisture, fingerprints, and other corruptions of the outside world. Big K could only be retrieved by a gathering of three custodians, each with a different key.
Forty identical sister copies were shipped abroad to calibrate kilograms worldwide. The cylinders were reunited only three times for comparison. Each time, Big K and its twins were delicately wiped with alcohol and ether, steam-cleaned, and weighed. In 1992, scientists were disturbed to discover that Big K had somehow become lighter than its siblings.
Nobody is certain how it happened. And because Big K’s mass is measured relative to the other cylinders, it’s not clear whether it’s been losing weight or its doppelgangers around the world have been gaining weight. Perhaps a gradual build-up of contaminants is the culprit, such as mercury leaking from lab thermometers. One researcher even sought to solve the problem by instituting a new, standardized method for cleaning the cylinders, using UV light.
"You are afraid to touch it," says Stephan Schlamminger, lead scientist of the electronic kilogram program at the U.S. National Institute of Standards and Technology. “You may rub some atoms off or something."
The Mole People
Although the gap in weight is small—about 50 micrograms, or roughly the size of a small grain of sand—this instability is unacceptable in precise fields like medicine or engineering, where tiny differences can cause immense problems.
More importantly, this isn't just a question of mass. Many other phenomena—force, pressure, and energy—rely on the kilogram for calculating measurements. A constantly fluctuating kilogram would send other familiar units, like watts and volts, tumbling into uncertainty.
Finally, in 2011, the CGPM agreed, by a unanimous vote of all 55 delegates, to redefine the kilogram according to a physical constant. The target date is 2018. One of the methods being developed would replace Big K with a measurement based on Avogadro's number.
Avogadro's number, named for a 19th century chemist, is also known as a mole—from the Latin moles or heap. It’s a term that represents a grouping of atoms or molecules. Just as a dozen eggs means 12 eggs or a ream of paper is 500 pages, a mole of atoms is around 602,214,082,000,000,000,000,000 individual atoms.
How large a number is that? "Avogadro's number of marshmallows spread uniformly over the entire 50 United States would yield a blanket of marshmallows over 600 miles deep," noted an article in a 1978 issue of the Journal of Chemical Education.
Chemists use Avogadro's number to translate measurements at the atomic level into grams.
That relationship can be used to pin down the mass of a kilogram.
Counting to Avogadro
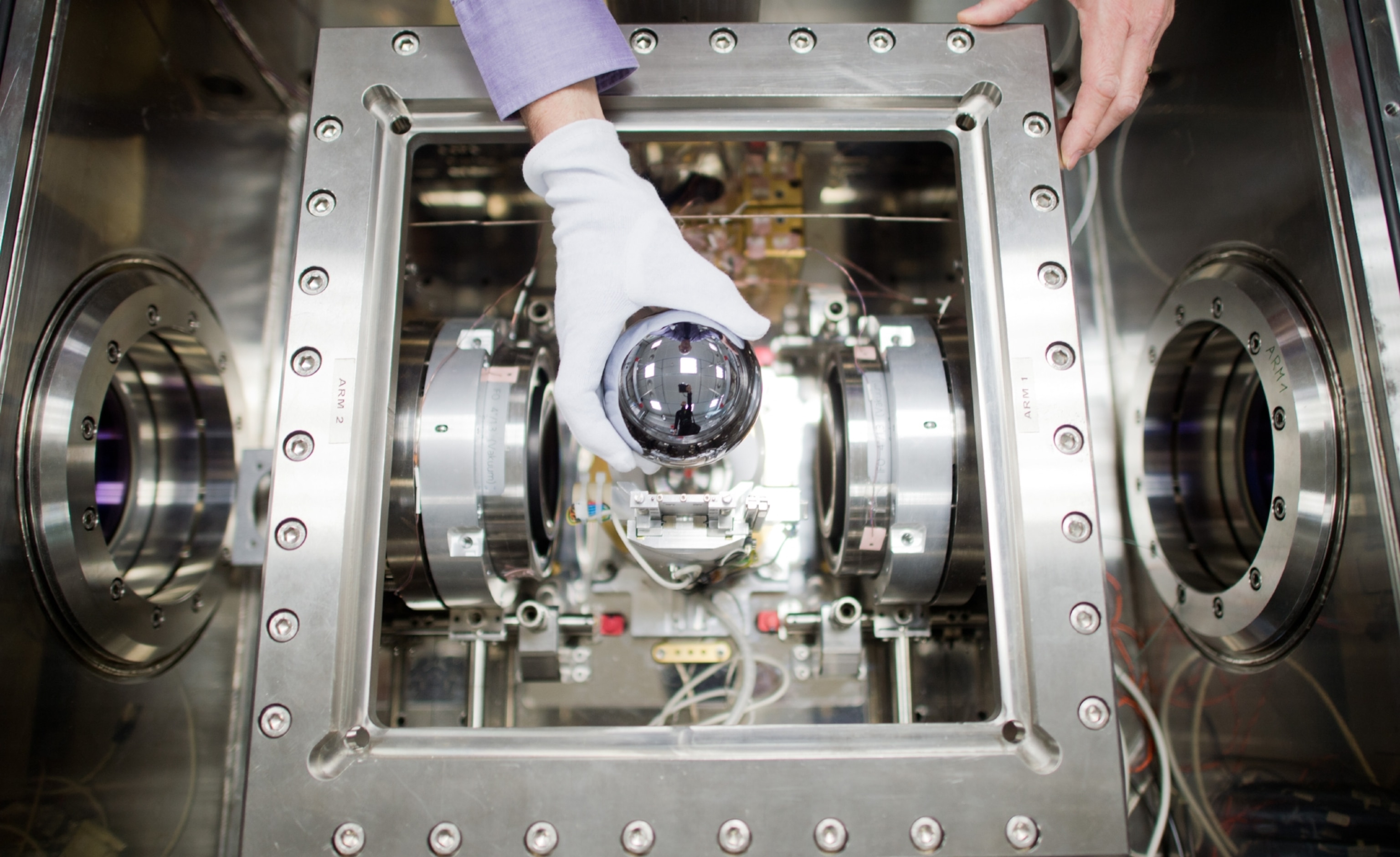
To work out Avogadro's number, researchers needed a substance that grows perfect crystals, where each atom takes up an identical amount of space.
That substance is silicon. Scientists counted the number of atoms in a reflective sphere of one kilogram of silicon. Like counting gumballs in a gumball machine, they measured the size of the shiny sphere and calculated how many atoms fit inside.
The result, published in the Journal of Physical and Chemical Reference Data, is the most accurate value of Avogadro's number of ever recorded, with an error of only 0.000000018.
In a few years, we might finally be able to retire Big K. Anybody need a paperweight?
Follow Maya Wei-Haas on Twitter