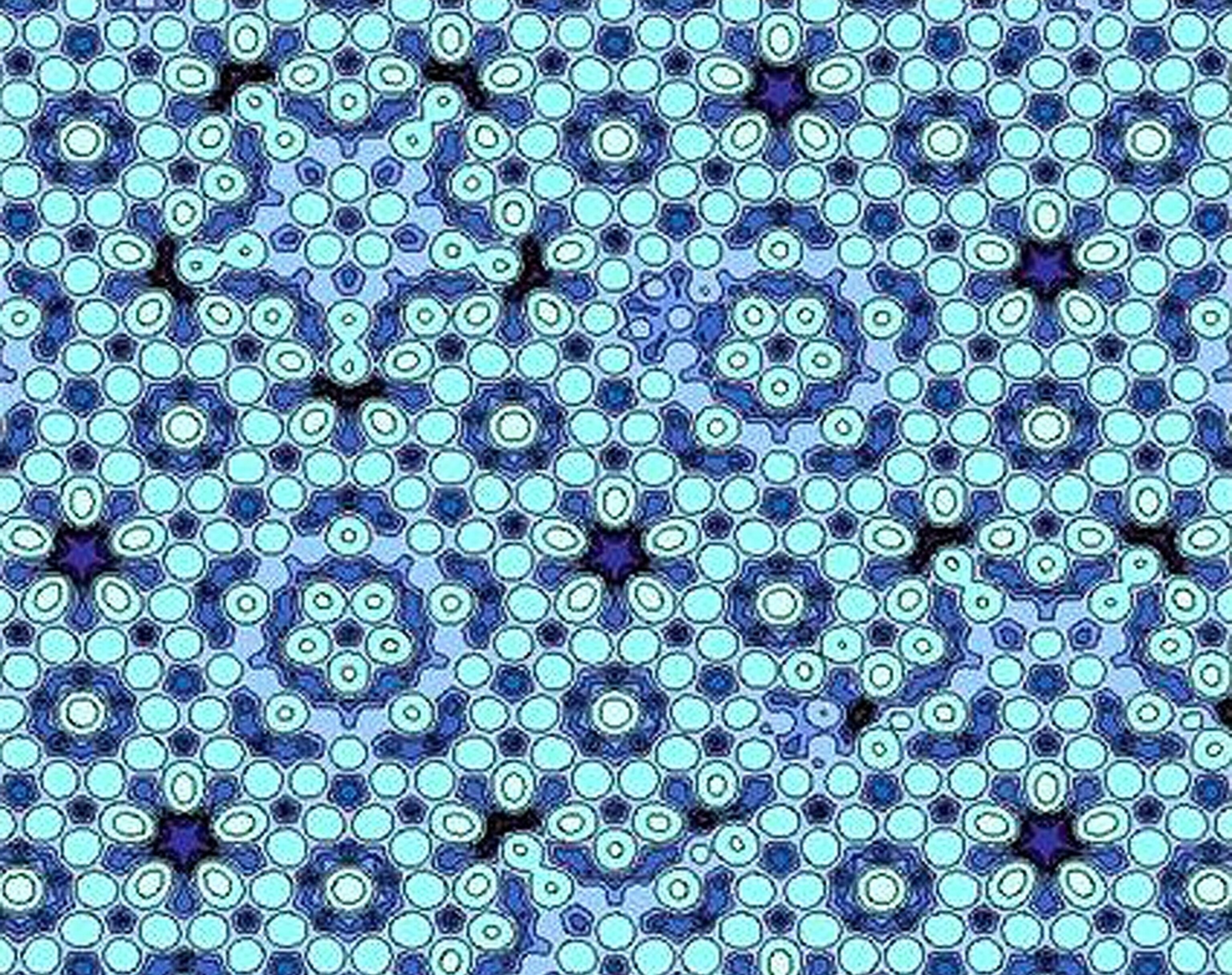



Quasicrystals: Tougher Steel, Better PansResembling mosaic tile, this atomic model shows a type of quasicrystal, a material whose atoms display a regular but nonrepeating pattern—once thought impossible in crystals. The discovery of quasicrystals earned Israeli scientist Daniel Shechtman the 2011 Nobel Prize in Chemistry Wednesday—and joins the list of Nobel-winning chemical discoveries with the potential to change the way we live."Quasicrystals were an unexpected state of matter when Shechtman discovered them. ... [and] there was initial resistance to the discovery," said Thomas Tritton, president and CEO of the nonprofit Chemical Heritage Foundation, based in Philadelphia, Pennsylvania. "Luckily, persistence [and] further research carried the day."Schectman, of the Technion-Israel Institute of Technology, glimpsed his first example of quasicrystals' "forbidden symmetry" in April 1982, when he was studying a metallic crystal made of aluminum and manganese under a microscope. The researcher spotted a unique diffraction pattern of concentric circles made up of ten bright dots, all at the same distance from each other. At the time, scientists thought a crystal could have only four to six such dots.Since Schectman's initial discovery, other quasicrystals have been discovered in nature and in the lab. One quasicrystal has been found in a kind of highly resilient steel now used in razor blades and surgery needles.Because of quasicrystals' unique physical properties, scientists are also experimenting with using the crystals in products ranging from diesel engines to frying pans."If you take an aluminum pan and cook something on it, the temperature reaches a high value quickly," and the pan heats unevenly, said Bassam Shakhashiri, president-elect of the American Chemical Society."If you use a pan with a quasicrystal coating, that helps a great deal in distributing the heat, because quasicrystals-even metal ones-are poor conductors of heat and electricity due to the way their atoms are arranged and bonded."Overall, Shakhashiri called quasicrystals "a great intellectual discovery ... and its potential to help society is just beginning to be seen."—Ker Than
Image courtesy U.S. DOE via AFP/Getty Images
Photos: Life-Changing Nobel Chemistry Breakthroughs
Quasicrystals today joined scores of Nobel prize-winning chemistry advances that have changed how we live—from radiology to neon signs.
Published October 6, 2011