Will Marijuana for Sick Kids Get Government to Rethink Weed?
The drug’s ability to reduce seizures in some children has softened opposition to research and may someday lead to changes in government policies.
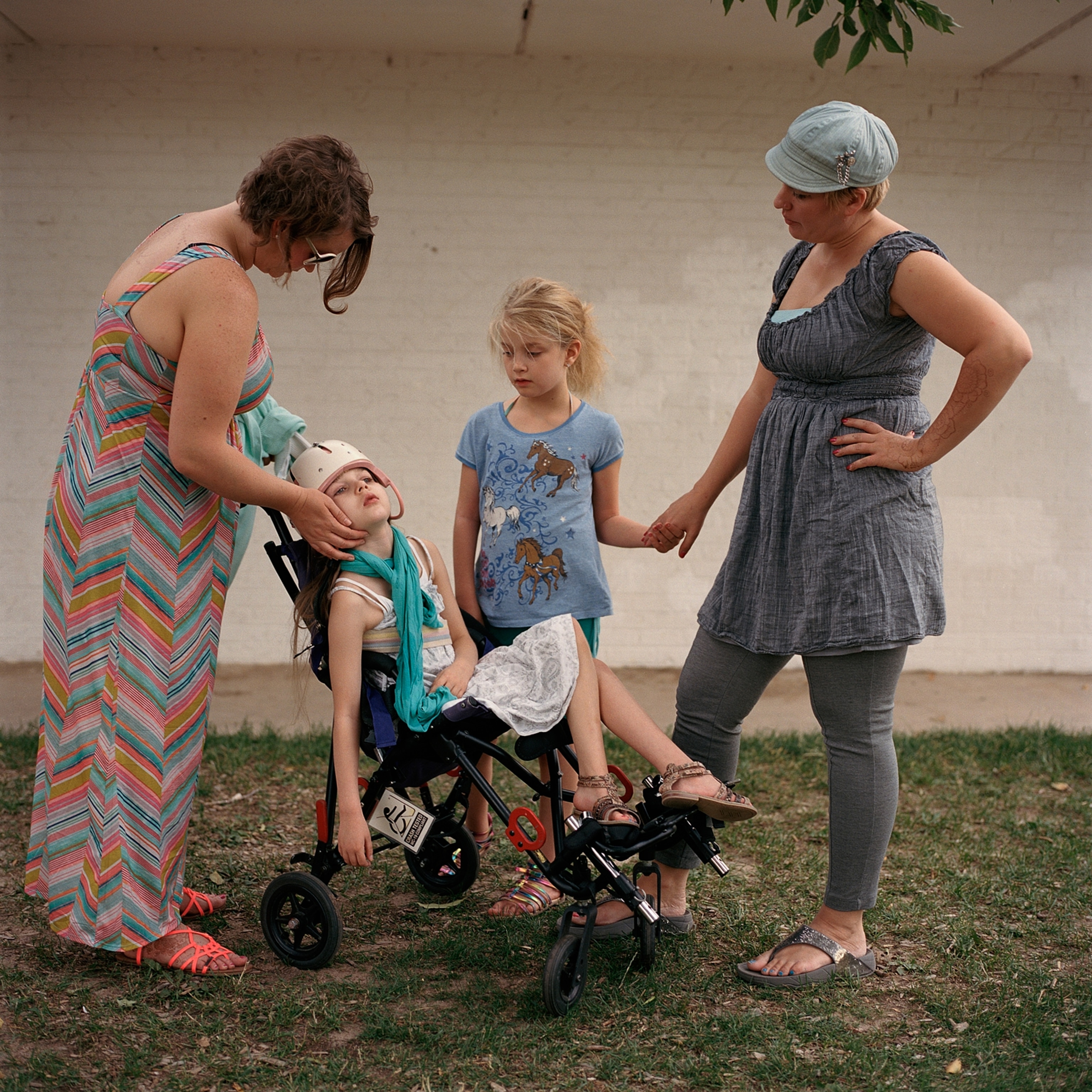
For years, opponents of legalizing medical marijuana have built their case on the most powerful of political maxims: Think about the children. But today it’s the suffering of children that might eventually compel the federal government to relax its stance.
Thousands of kids across the United States are afflicted with Dravet Syndrome and Lennox-Gastaut Syndrome, rare forms of childhood-onset epilepsy that can cause dozens, even hundreds, of severe seizures each day. Conventional drugs have been ineffective.
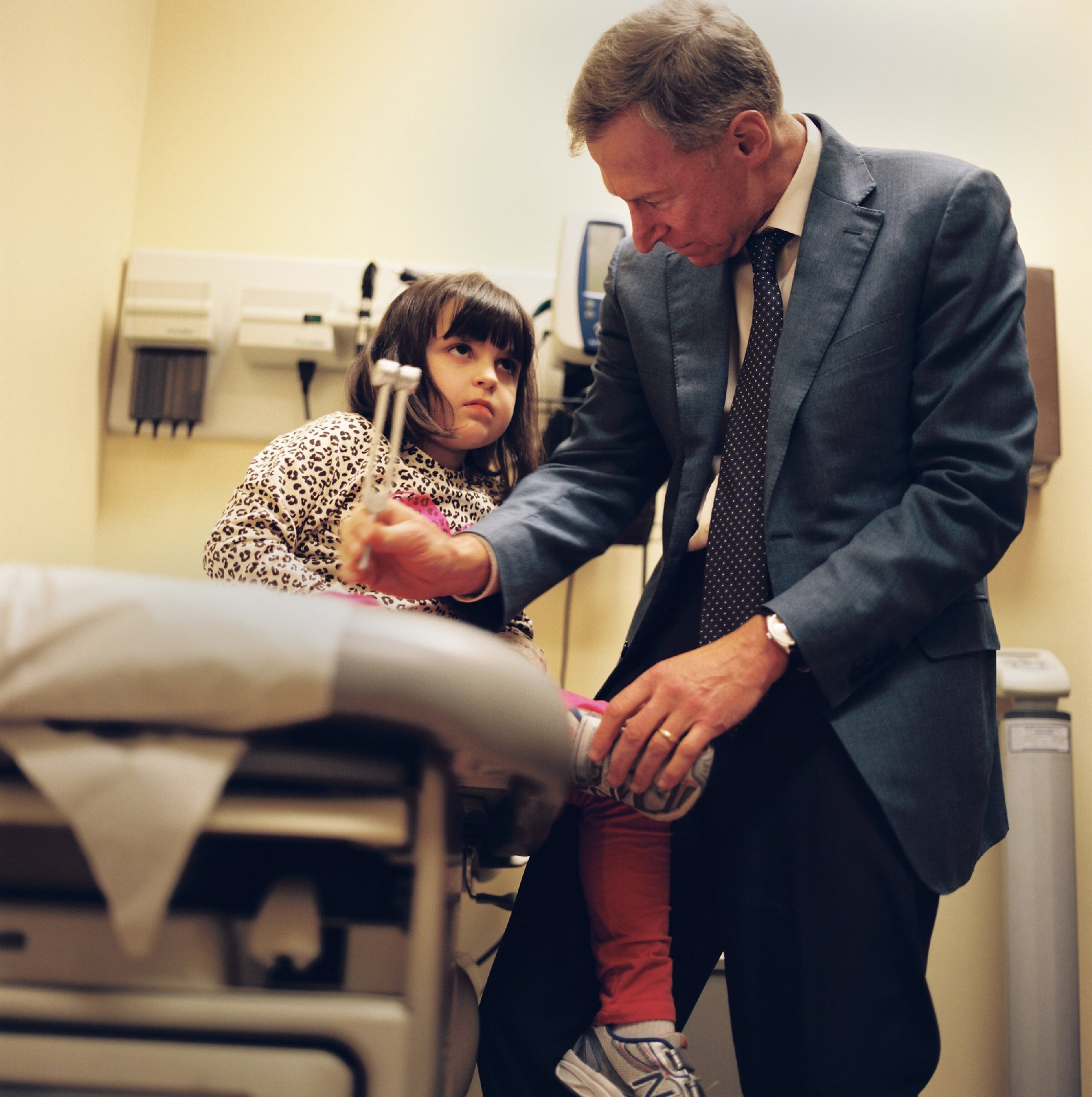
Last year, however, the FDA approved a clinical trial of Epidiolex, a drug made from cannabidiol (CBD)—one of 85 active chemical compounds, called cannabinoids, in marijuana. The initial findings were promising. After 12 weeks of treatment, 54 percent of patients experienced fewer seizures and 9 percent saw their seizures cease. The trial has already moved to a double-blind, placebo-controlled study. (Read about the new science of marijuana.)
In addition, scientists are stepping up lab research to better understand the mechanisms of CBD, which, unlike tetrahydrocannabinol (THC), is not psychoactive. Joseph Sullivan, the director of the University of California Pediatric Epilepsy Center in San Francisco, who was also one of the investigators in the Epidiolex study, says that one of the most significant developments driving this research is that the medical community is no longer lumping cannabinoids together.
“We now know that it is the cannabidiol alone that exhibits the most antiseizure effects,” he says, “and with some of the new genetically hybridized plants, we have the ability to get higher concentrations of cannabidiol without concomitant concentrations in THC.”
But while cannabidiol can’t get anyone high, scientists repeatedly confront legal and bureaucratic obstacles to their research. Since it is extracted from the marijuana plant, it is still classified as a Schedule I drug under the Controlled Substances Act. That places CBD in the same category as LSD and heroin—a drug with “a high potential for abuse” that “has no currently accepted medical use in treatment in the United States.”
Enabling Cannabis Research
The Obama administration or Congress could “reschedule” marijuana to a category that would acknowledge its potential medical benefits and reduce the restrictions on research. But John Hudak, an expert on the legalization debate at the Brookings Institution in Washington, D.C., believes that’s unlikely to happen soon.
Although opinion polls show growing support for medical marijuana, even in conservative states, “there is still a lot of fear about marijuana that exists in the public,” Hudak says. “A vote in favor of rescheduling marijuana could very easily turn into an attack ad in the next campaign: ‘Politician A is making it easier for your kids to get high.’”
And while the administration could reschedule marijuana on its own, “this is not an area where they feel like engaging in a fight with Congress,” Hudak says. In general, Hudak believes there’s not much enthusiasm among elected officials to spend political capital on medical marijuana because “only a small community is very passionate about it.”

If anything, progress on the issue could stall in the coming months. Loretta Lynch, the new U.S. attorney general, revealed during her Senate confirmation hearings that she is less reform-minded on marijuana legalization than her predecessor. And those who see the recent departure of DEA Administrator Michele Leonhart as an opportunity for change should be prepared for disappointment. Caught between old-school drug warriors and reformers, President Obama opted to name an acting DEA chief who will likely maintain the status quo for the remainder of the administration, Hudak notes.

Changing Opinions on Pot
Still, Hudak believes that if demand for cannabidiol research increases, it could have a lasting impact by reducing marijuana’s stigma. “There is a lot of ignorance in the political community about CBD,” he says. “I think people, particularly opponents of reform, think of marijuana as a rolled joint and that's it. When, in reality, it's a very diverse product.”
From Hudak’s perspective, the interest in cannabinoid medicine signals a shift among medical professionals—whose minds, he says, can be “more difficult to change” than those in Congress. Earlier this year, for the first time, the American Academy of Pediatrics called for the rescheduling of marijuana to make it easier to develop treatments for children "with life-limiting or severely debilitating conditions for whom current therapies are inadequate."
Sullivan, a pediatric neurologist, doubts the FDA would have granted permission for Epidiolex trials were it not for the “social media buzz” stirred two years ago when celebrity doctor Sanjay Gupta reversed his opposition to medical marijuana while working on a high-profile CNN documentary that highlighted CBD. And Hudak says that, as more of the medical community throws its support behind marijuana-derived treatments, it will become easier for politicians to sell constituents on reform: “They can say, ‘Listen, we’re not big fans of marijuana, but the last thing we want to do is come between you and your doctor.’”
But where do federal agencies currently stand? One way to gauge their thinking is to look at what’s going on at the University of Mississippi, the site of the only cannabis farm sanctioned by the government. The facility is overseen by the National Institute on Drug Abuse (NIDA), which places orders for strains it wants for research.
Last month, the DEA signed off on a NIDA request to more than triple federal marijuana cultivation from its previously stated 2015 quota of 276 pounds (125 kilograms) to 881 pounds (400 kilograms), explaining that “research and product development involving cannabidiol is increasing beyond that previously anticipated.”
Also, for the first time, the government contract with the university includes an authorization to directly manufacture cannabidiol (up to 220 pounds, or 100 kilograms). The contract includes an option to scale up overall marijuana production from as low as 1.5 acres to as high as 12 acres.

A Door Cracks Open
Mahmoud ElSohly, who runs the NIDA Marijuana Project at the University of Mississippi, cautions against reading too much into these figures. “NIDA has been criticized over the years for not having this, not having that, when an investigator needs something,” he says, “so they just wanted to be proactively ready to respond to the needs of the research community.”
Hudak shares ElSohly’s view, though he also thinks it’s an indicator that the administration is thinking long term, preparing for a time when the federal government might relax its restrictions. “One of the best characteristics of the president is his willingness to lay the groundwork for change that he thinks will eventually happen, even in the face of not yet being able to effect that change,” he says.
Meanwhile, GW Pharmaceuticals, the British company that manufactures Epidiolex, has announced that the FDA has approved initial, preclinical studies to see whether cannabidiol can be used to treat newborn children with neonatal hypoxic-ischemic encephalopathy—a type of brain injury caused by lack of oxygen during birth.
At present, there is no FDA-approved drug for those children, who suffer from several disabilities, including multiple seizures. But researchers speculate that CBD could be an especially useful treatment, since it not only controls seizures but also has potent anti-inflammatory properties that could mitigate the effects of brain damage.
With this latest announcement, it’s increasingly clear the door has cracked open for FDA-approved cannabinoid research. For now, these studies are limited to rare disorders without effective treatments. But other scientists hope to expand research on how cannabis extracts could be used to treat Alzheimer’s disease and schizophrenia. If such research shows promising results, the federal government could find itself under pressure to reschedule cannabis and recognize the drug’s medical value.
Follow Mark Strauss on Twitter.